Marketing hospital textiles in the EU market
By Janelle Bentz, study director at Nelson Laboratories 22 June 2017Complications after surgical procedures are a major cause of death and disability worldwide. These complications are often caused by bacteria that are typically safe, but they lead to healthcare acquired infections (HAIs) when they breach the sterile field during operation. The sterile field is maintained in the surgical suite by the use of protective barriers, such as surgical drapes, gowns and clean air suits.
Surgical drapes are used to cover parts of the patient during surgery, and surgical gowns and clean air suits are worn as personal protective equipment (PPE) by operating room staff. These barriers are used to contain liquids, particulates and other contaminants which might be present during surgical procedures.
Qualifying products to market in Europe
The European standard, EN 13795, is a harmonised standard in the European Union and is in place to make sure that barriers used during surgery have demonstrated they block the passage of pathogens to the patient. Products that are tested and have shown they meet this standard fulfil the requirements for either “standard performance” or “high performance” claims. Products that meet the criteria for either of these claims are able to apply for the CE marking, which is necessary for products that are marketed in the European Union.
This marking, which literally means “European conformity”, is the manufacturer’s declaration that their product complies with the essential requirements set out by the new European Medical Device Regulations (MDR). Products that meet the minimum requirements for EN13795 meet the requirements for the CE marking, and comply with the MDR. The MDR outlines guidelines for the safety, quality and usefulness of a device for its intended purpose, and it suggests items to be included in the clinical evaluation report (CER).
The CER can include a literature evaluation; a description of the device or product; the scope of the evaluation included therein; some risk management documents; an analysis of all alternatives to the proposed product/treatment; a discussion of equivalence and differences in clinical, technical and biological areas; and a plan for post-market surveillance. Testing that complies with the methods outlined in EN13795 proves that a device adequately meets the specific performance requirements outlined in the standard, and test results should be included in the CER. Continual testing may also be included in the post-market surveillance section, in order to prove continued efficacy of the product.
EN13795 standard
In 2011, a new revision of the EN13795 standard was released. This version united three parts, which described characteristics to be evaluated, test methods and minimum requirements from separate parts into one document, thus harmonising all three parts into one standard. EN13795 consists of a suite of tests that examines the barrier properties, cleanliness and strength of products that are meant to protect patients and clinical staff from infection. Each of these properties is important in maintaining a clean surgical environment, and EN13795 sets specific performance requirements that are measured using certain tests outlined in the standard. Barrier testing includes resistance to dry and wet microbial penetration, and resistance to liquid penetration. Cleanliness evaluates both biological burden and particulate shedding. Strength measures bursting and tensile strength in both wet and dry states.
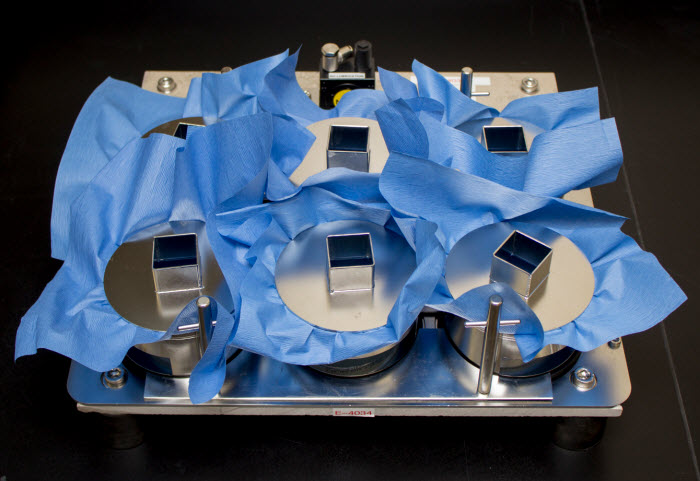 Penetration testing ensures water or other contaminated liquids or materials are blocked by the barrier. Images courtesy of Nelson Labs.
Penetration testing ensures water or other contaminated liquids or materials are blocked by the barrier. Images courtesy of Nelson Labs.
Barrier testing included in EN13795 evaluates the ability of bacteria to penetrate the drape, gown or clean air suit material in both wet and dry states, and it sets performance standards to ensure that bacterial penetration is managed. While most bacteria are normally harmless on the skin, they can cause serious infection if they enter a surgical incision. Liquids found in surgical suites are common carriers of bacteria. Fluid resistance is necessary, not only to ensure that surgical fluids are contained, but also to ensure that the operating room staff is protected from exposure to bodily fluids. Dry microbial penetration resistance is important as bacteria can also be carried on dry particles such as dead skin cells, which are actively shed by staff as they perform various duties in the operating room. Performance criteria are included in the standard to ensure the materials used for the drapes, gowns and clean air suites are protective against bacterial ingress from both wet and dry sources.
Cleanliness testing is essential to safeguard that the sterilisation technique used for surgical woven and nonwoven materials is adequate, and to guarantee that items used in a sterile surgical environment do not contaminate the environment with particulate matter. Microbial cleanliness testing, also known as bioburden testing, measures the level of microbes present in a product after the manufacturing process is complete. If this testing is performed prior to sterilisation, information from test results can be used to calculate effective doses or times for specific sterilisation techniques. Testing performed after sterilisation can be used to validate the sterilisation technique. Particulate cleanliness testing measures the number of small particles released during stress to the material. The standard sets a cleanliness threshold that must be met since particulate matter can be dangerous if it enters a patient wound.
 Cleanliness testing ensures barriers are free from harmful microbes and lint particles
Cleanliness testing ensures barriers are free from harmful microbes and lint particles
Strength testing described by this standard measures both tensile strength from force in one direction and resistance to bursting when pressure is applied in multiple directions. This testing is important to certify that surgical gowns and drapes can withstand the pressures exerted on them as they are worn, leaned on, moved, pulled and otherwise manipulated during procedures. Minimum levels for strength are outlined and must be met in order to comply with the standard’s requirements. Having a barrier break during surgery is an undesirable situation, and can lead to exposure for the patient and the surgical staff. Strength testing is in place to help minimise this risk by guaranteeing that gowns, drapes and clean air suits used for medical purposes have proven minimum bursting and tensile strength.
The European Union is harmonised in the adoption of EN13975 for surgical gowns, drapes and clean air suits as it has established the minimum requirements needed to protect patients, surgical staff and the operating environment from contamination. The standard clearly outlines the testing that needs to be performed along with performance criteria, but the standard does not specifically spell out what are considered critical and less-critical areas of the products. According to the standard, a critical area is the “product area with a greater probability of being involved in the transfer of infective agents to or from the wound,” and the less-critical area is the “product area less likely to be involved in the transfer of infective agents to or from the wound.”

Strength testing ensures barriers can withstand the hardships of being manipulated during use
without tearing or bursting
Manufacturers are encouraged to consult with industry experts to determine the criticality of the sites on the gowns, drapes and clean air suits, and to determine the appropriate testing plan for their products. Manufacturers whose products adhere to this standard meet the CE marking and MDR requirements and are, therefore, eligible to market their devices in Europe.
Have your say. Tweet and follow us @WTiNcomment
RELATED ARTICLES
-
Sewing thread invisible to infrared cameras
- Durak Tekstil
- WTiN
-
Regenerative materials transform outerwear
- Abigail Turner
- WTiN
-
Next-generation materials roundup
- Abigail Turner
- WTiN